healthcare
+ medtech
Medical Software Development
We design and develop custom HIPAA-compliant and FDA-ready medical software and applications for telemedicine, in-office use, training, or in-the-field environments.
Our team specializes in Software as a Medical Device (SaMD), clinical support tools, and healthcare-focused digital platforms that improve outcomes and meet strict regulatory standards.

Create, Update, or Overhaul Medical Software or Apps
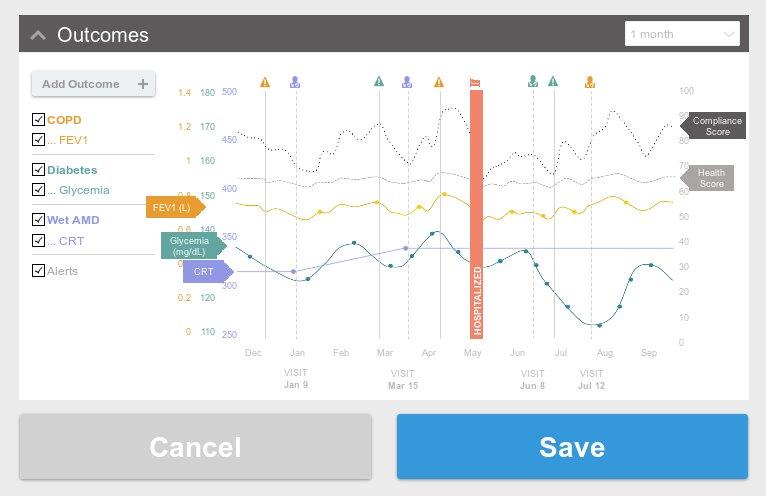
Custom biomedical software requires not only technical expertise but also deep familiarity with regulatory pathways such as HIPAA, FDA 510(k), IEC 62304, and SaMD classifications. Whether you’re navigating the development of a Class I SaMD solution or integrating a novel feature into an existing EHR, we can help.
We’ve partnered with clients at all stages of the product lifecycle—from early prototypes to post-market updates and compliance support. Our team is often called upon to revive struggling projects, ensuring continuity and quality, or to lead full development cycles from concept through deployment and regulatory submission.
Art+Logic engineers and designers have delivered software for MedTech innovators, hospital systems, research institutions, and healthcare startups across the country. We build software and companion apps for connected medical devices, cloud-based platforms, and mobile diagnostics—and we never outsource or offshore our work.
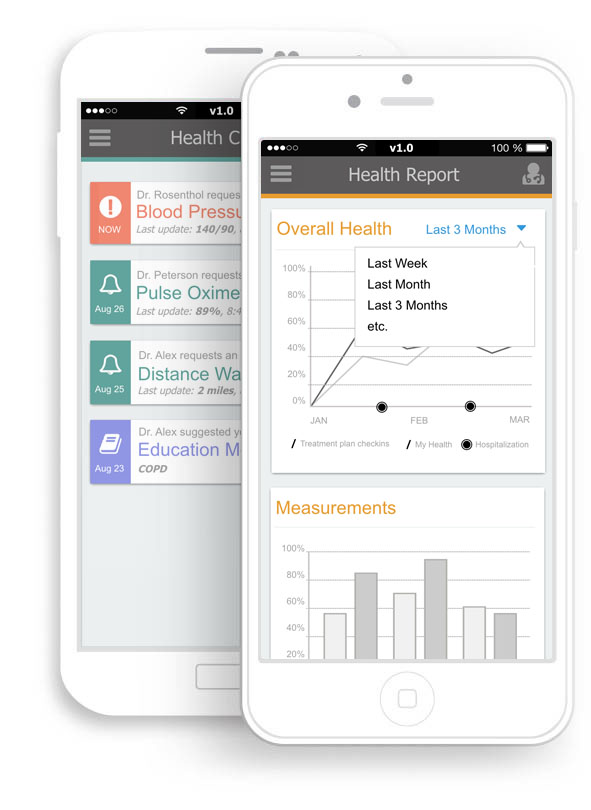
Biomedical Software Apps
We design and develop apps and platforms to:
Improve patient and provider communication
Help patients manage chronic or acute conditions
Facilitate field data calibration for medical equipment
Track and manage patient medications securely
Consolidate and interpret large sets of clinical data
Deliver cost-saving solutions through automation and analytics
Support remote physician consulting and virtual care models
Engage patients with custom health platforms and reminders
Manage patient medical records in compliance with HIPAA standards
Build pharmaceutical tracking and management tools
Route patient data through secure organizational workflows
Deploy to the Apple App Store, Google Play, or enterprise platforms
Biomedical Software Solutions
Field Data Calibration
Patient Data Collection
Physician/Patient communications
Medical Data Consolidation
Instructional + Training Apps
SaMD (Software as a Medical Device) architecture and compliance
FDA 510(k) submission readiness
Data interoperability (FHIR, HL7, DICOM)
Risk classification and documentation support
“It’s been a pleasure working with Art+Logic’s app development team to build the Emergency Medicine Residents’ Association (EMRA) PressorDex app.
As a physician who works in a high-intensity work environment like the emergency department or intensive care unit, a clinical application’s content must be clear, reliable, and easy to use.
The developers at Art+Logic have been extremely responsive in making sure that our app’s functionality meets these needs as well as what our users want.”
HIPAA compliance

HIPAA Compliance
Our developers are fluent in the HIPAA Privacy Rule and its implications for software architecture, data storage, and third-party integrations. We design systems that protect patient data, meet legal requirements, and stand up to evolving compliance audits.
As medical software becomes increasingly connected—from remote monitoring tools to patient engagement apps—security, privacy, and trust are more important than ever. Our team stays current with HIPAA updates, as well as guidance from the FDA, HHS, and State Deparetments of Public Health on SaMD, digital health tools, and healthcare software in the era of AI and remote care.
For current HIPAA and digital health regulations, visit the US Department of Health and Human Services.
New Website and Branding
let's talk
"*" indicates required fields